Differences between Alpha and Gamma Aluminum Oxide Nanoparticles
Differences between Alpha and Gamma Aluminum Oxide Nanoparticles
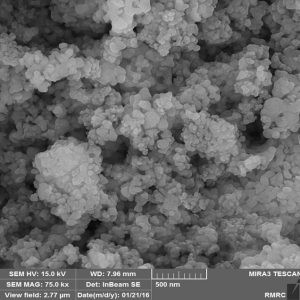
Structural difference: in terms of structure, the alpha-alumina is trigonally structured (R-3c) with an ABAB arrangement of planes of oxygen along the c-way. The Y-alumina on the other hand has a cubic shape and group name-Fd-3m coupled with an FCC. It has an ABCABC oxygen stacking. Unlike the alpha-alumina, it has vacant spaces on AI (iii) posts. Again, unlike the alpha-alumina that occupies 2/8 of the octahedral interstitial positions, the Y-alumina occupies not only the octahedral position but also the tetrahedral positions, though the level of occupancy remains a subject of dispute.
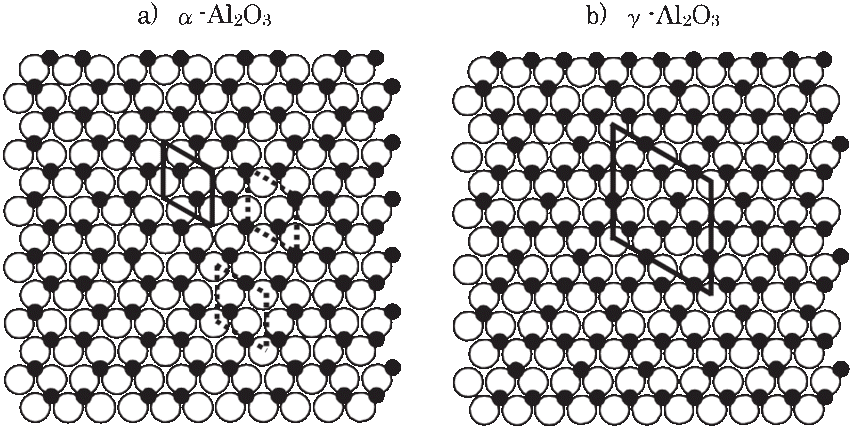
Model structures of α -Al2O3(0001) and γ -Al2O3(111). Rhombuses in the structures show the unit cells
Temperature: both of them also vary in terms of temperature. When exposed to HGH temperature, both the alpha and gamma-alumina structures or phases with temperatures ranging from 1050 to 1100 and 400 to 600 °C, respectively. Based on XRD calculation, it is shown by the Scherer equation that while alpha alumina has a crystallite shape of about 33 nm, the Y-alumina lies within the range of 5.0-10.0 nm. Again, the alpha alumina observes its highest surface area of 266.om²/g at 400 °C and lowest of 5.0m²/g at 1100 °C with the overall pore volume as 600 °C. Again, porosity decreases at highest temperatures. The Y phase has a low melting room temperature and can be used in the manufacturing of plastic sapphires using thermal melting methods. Due to its thermal properties, alpha phase can be used in the production of plastics, ceramics, and other factory products. They equally help to improve density ratio in ceramics and serve as anti-fatigue thermal resistance. Also, since the alpha phase has a high performance, it is widely used in the production of synthetic rubies, aluminum garnets, and other fabrics.
Specifications: Both the alpha and gamma aluminum oxide also differ in terms of specifications. The following are the specification of Alpha alumina: amorphous, colour-white, crystal form-alpha, purity-99.9% APS-30 to 60 nm, 100-150nm,.150-200nm and 500 respectively. In gamma aluminum oxide, the form is nanopowder, color-white, purity-99.97%, size-20 to 30nm.
Differences Between Alpha and Gamma Al2O3 Nanoparticles: Experimental approach
Perhaps, it is a better approach to explaining their differences through the use of experiments. The purpose of the experiment will be to not only compare the impact of different aluminum oxide phases on thermal conductivity of oil but to establish how each phase works differently.
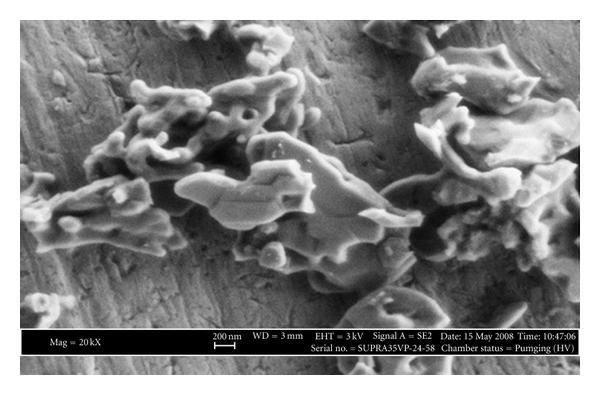
In the experiment, Effect of Al2O3 phases on the enhancement of thermal conductivity and viscosity of nanofluids in engine oil, a sol-gel method was used in synthesizing Alpha- aluminum oxide. To achieve this, about 15 grams of aluminum nitrate with a 99% purity was melted in deionized water of 1000 cl. A solution containing 20 wt% of carbon black and ethyl alcohol was added and stirred into the nitrate solution. The mixture was stirred for approximately two hours by oven heating to form a dry gel. The obtained result was then heated in an oven for about ten minutes, with the specific surface area measuring up to 48 (m²g–¹).
The resulting product showed nanoparticles with an average diameter of 20nm and density, 3.96 (gr cm–³). The aluminum oxide gamma obtained had a density of 2.8 (gr cm–³). Engine oil was selected as the nanofluid. To achieve this, powders were added to the oil to obtain fluids ranging from 0.1-3wt% concentrations. Afterward, the concentration was stirred for 30 minutes at 100 °C.
The process was followed by an ultrasonication of the nanofluids for 30 minutes in order to remove particles from the fluid. The results showed that thermal resistivity was increased as it increased the solid-phase content. It was also shown that about 31.47% and 37.48% improvement in conductivity was obtained for fluids with alpha- aluminum oxide and y- aluminum oxide accordingly, which originated from their distinction between alumina conductivity and liquid means (being 35 (wm–¹k_¹) and 0.145 (wm–¹k–¹). The reason for the difference is the result of a higher surface area as against alpha- aluminum oxide of the same molecular size. The result further demonstrated that the surface area of the y- aluminum oxide plane is by far greater than the alpha- aluminum oxide, while its density is lesser than alpha- aluminum oxide.
However, in terms of molecular size, both are similar, being around 20 nanometers. Thus, the difference in fluids conductivity is not unrelated to the structure of the solid particles and their porous nature. Another reason for the increased conductivity can be seen in the concentration that the Y- aluminum oxide is higher due to the density.
Another notable difference is in the viscosity behavior. A linear behavior gave rise to new tonic behavior in fluids. Thus, it can be established from the above experiment that thermal resistance is facilitated by the addition of aluminum nanoparticles. By adding 3 wt% of Y- aluminum oxide, an improvement of 37.49% was recorded in the property.